Chronic Myeloid Leukemia, BCR-ABL1 positive
Clinical features
- Incidence of 1-2 cases/100k population.
- Acute radiation may be a risk factor, but unlike other MPNs, there is little inherited 3predisposition.
- Insidious onset. Most are either asymptomatic (~50%), or present with some combination of B symptoms and palpable splenomegaly (~50%).
- ~5% are diagnosed in blast phase.
- Natural history without treatment is progression to accelerated/blast phase in 3-5 yrs.
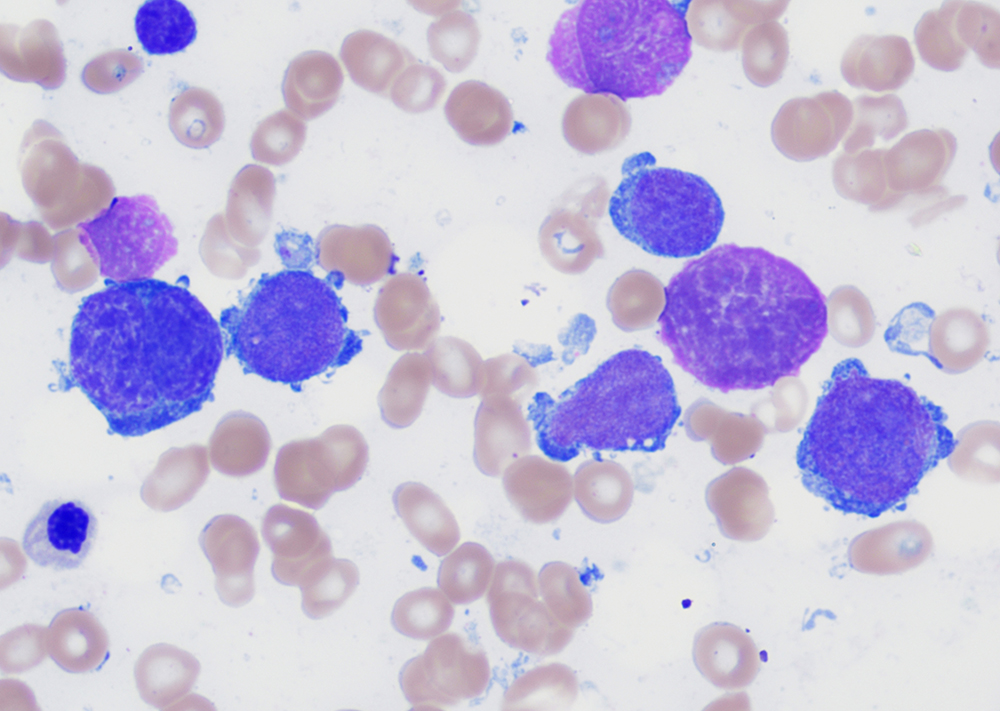
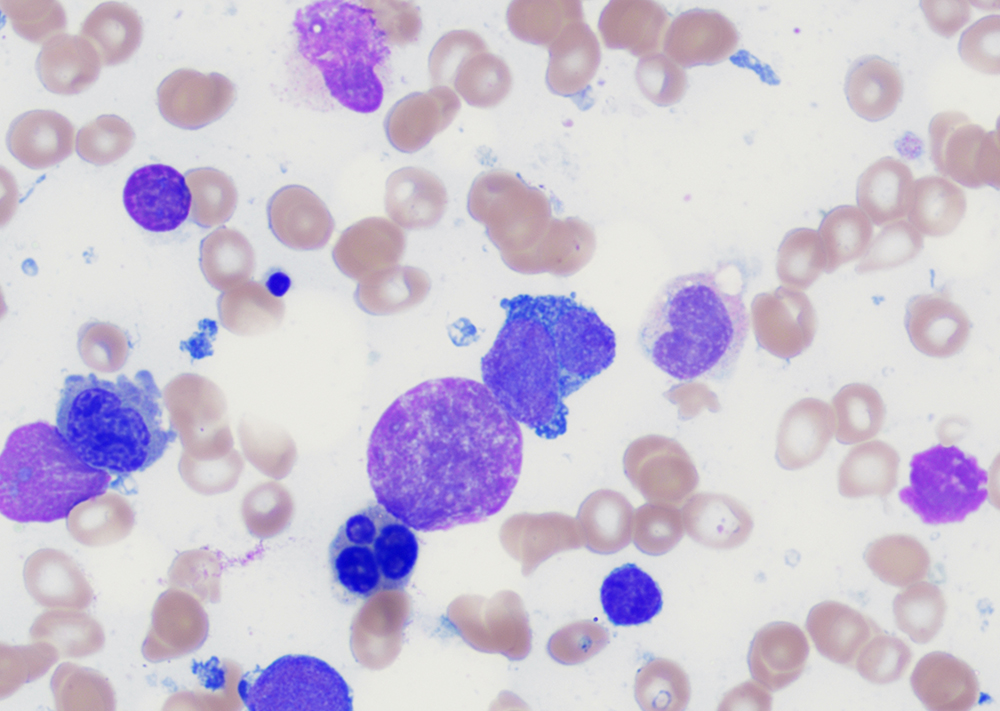
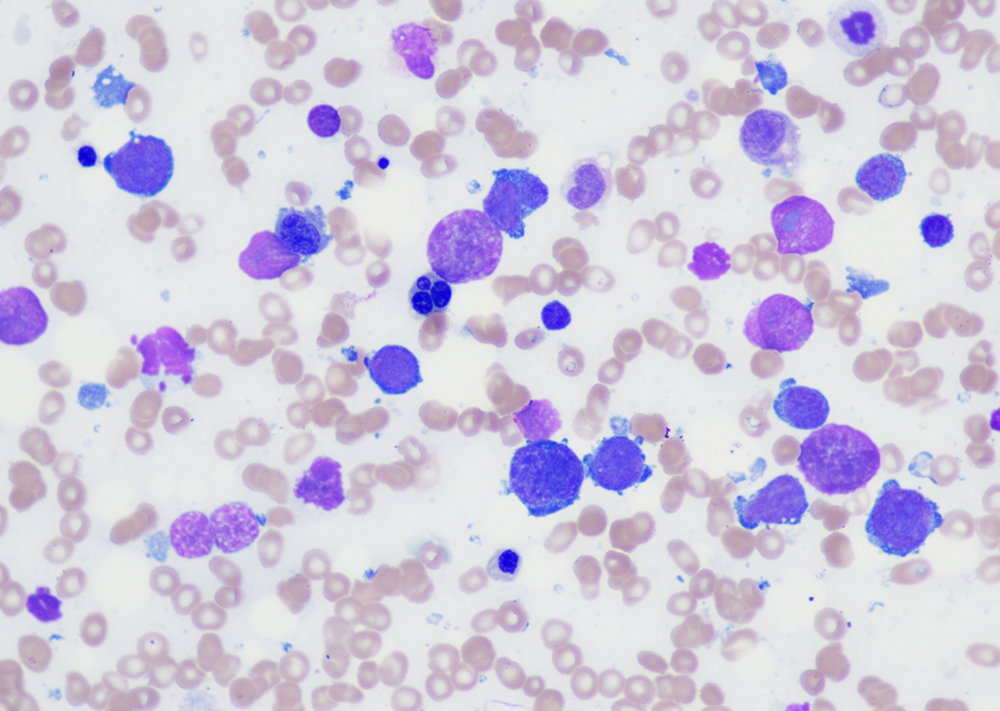
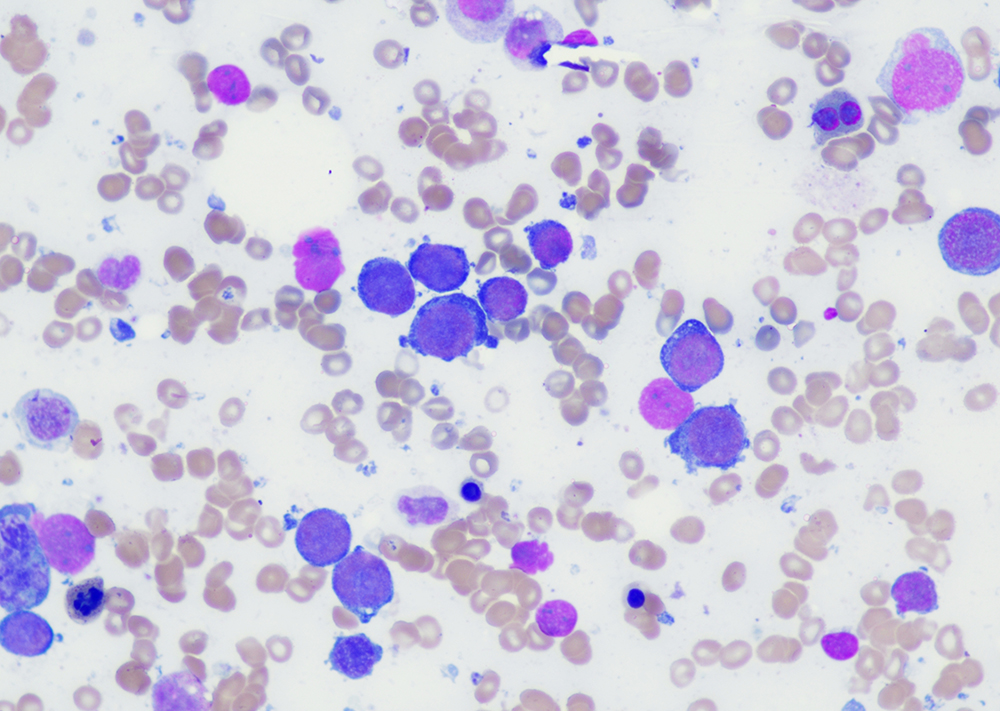
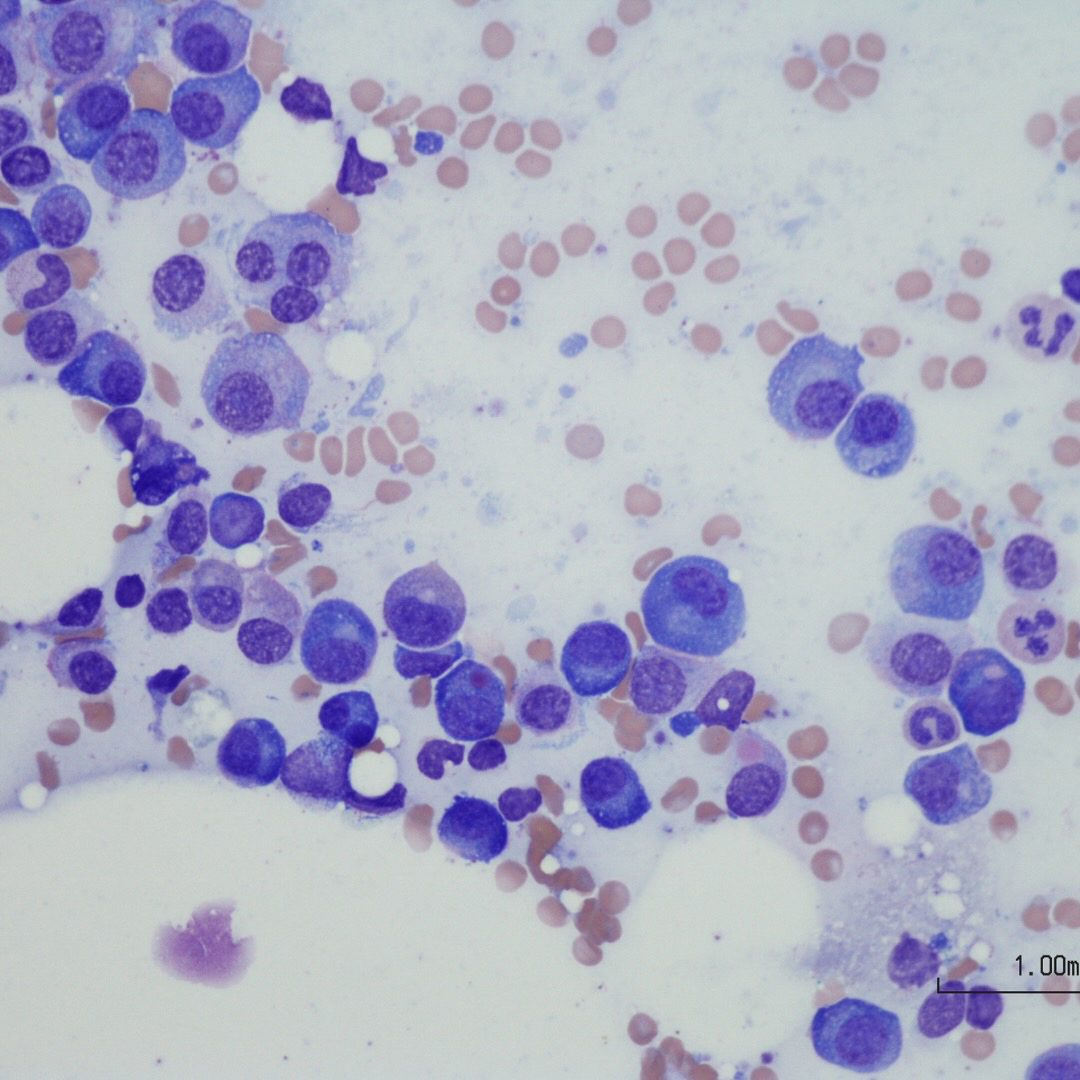
Morphologic features
- Peripheral blood:
- Leukocytosis with myelocyte bulge, basophilia, and eosinophilia. Children often have higher WBC counts than adults.
- Dysplasia is absent.
- Bone marrow:
- Although diagnosis can be made on peripheral blood, bone marrow aspiration is essential for confirming the phase of disease. Biopsy is not necessary but is recommended.
- Hypercellular marrow with granulocytic hyperplasia and left shift.
- Small “Dwarf” megakaryocytes with hyposegmented nuclei; many cases also show megakaryocytic proliferation.
- Increased eosinophils and basophils.
- Pseudo-Gaucher cells (non-specific).
- Moderate to marked reticulin fibrosis may be present and is often associated with splenomegaly and clustered small megakaryocytes.
Disease phases and progression
| Criteria | Additional features | |
| Chronic phase (CP) | Does not meet criteria for accelerated or blast phase. | Blasts are usually <5%. |
| Accelerated phase (AP) | Any of the following, despite therapy:
– Persistent/increasing WBC count (>10x10E9/L). – Persistent/increasing splenomegaly. – Persistent thrombocytosis (>1000x10E9/L), or thrombocytopenia (<100x10E9/L) not due to therapy. – ≥20% basophils in peripheral blood. – 10-19% blasts in blood and/or bone marrow. – Evidence of an additional clonal cytogenetic abnormality in Ph+ cells (either present at diagnosis, or which arises during therapy). |
Provisional response-to-therapy criteria for AP include resistance to 1st TKI, any resistance to 2 sequential TKIs, and ≥2 BCR-ABL mutations. |
| Bast phase (BP) | – ≥20% blasts per WHO (some guidelines use ≥ 30%) in blood or bone marrow.
– Extramedullary blast proliferation. |
Most cases have myeloid lineage; 20-30% of cases show lymphoid lineage (usually B-cell).
The finding of any number of lymphoid blasts should raise concern, and focal larger sheets of blasts in bone marrow can be considered equivalent to BP. |
Immunophenotype
- Main role of immunophenotyping is determination of blast lineage.
- Expression of CD7 by myeloid blasts may have poor prognosis.
- Most lymphoid BP cases are pre-B, with expression of TdT.
- Expression of lymphoid antigens in myeloid BP, and vice versa, is common.
Genetic Features
- 90-95% have t(9;22)(q34.1;q11.2) resulting in the Philadelphia chromosome (der(22)) and the formation of BCR-ABL1 fusion gene and fusion protein with constitutive tyrosine kinase activity.
- Remaining cases have variant or cryptic translocation.
- BCR-ABL1 breakpoints/isoforms:
- The major breakpoint (p210 isoform) is seen in most cases
- The minor breakpoint (p190 isoform) is seen in small amounts in most cases of CML, and is sometimes associated with monocytosis. This is the isoform most often present in Ph+ B-ALL.
- The p230 isoform is associated with neutrophilia and thrombocytosis.
- Mutations in the BCR-ABL1 kinase domain can lead to resistance to 1st generation TKIs, less so to 2nd generations.
Therapy, prognosis, and outcome
- Treated with 1st generation TKIs (imatinib) and 2nd generation TKIs (nilotinib, dasatinib, bosutinib, ponatinib).
- The most important prognostic factor is response to therapy.
- 10-year overall survival has improved to 80-90% with the advent of TKIs; approaching that of the normal population.
References (APA style)